Welcome
8B Science!
This year we will study a range of subjects from Newton's Three Laws of Motion, magnetism, waves and optics, fossils, and much more!
Please have one composition notebook dedicated to your science class. A pencil, graph paper, and loose paper are also a must-have. Optional but very helpful supplies to have of your own (I will have class sets but it's so nice to have your own if possible!) are colored pencils, a wooden ruler (metric, meaning centimeters), and scissors.
NOTE: Lesson plans may be altered at any time due to learners' specific needs.
Posts

Example for project

Example for project

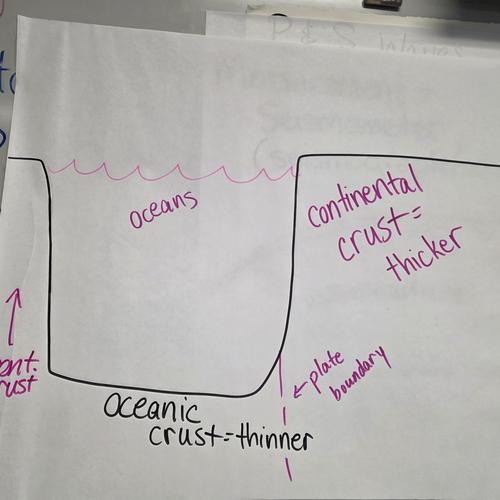
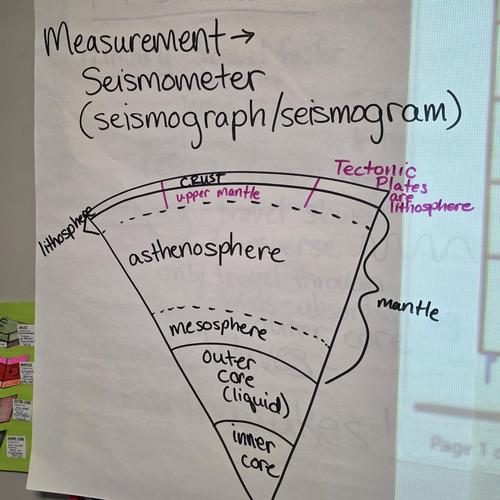
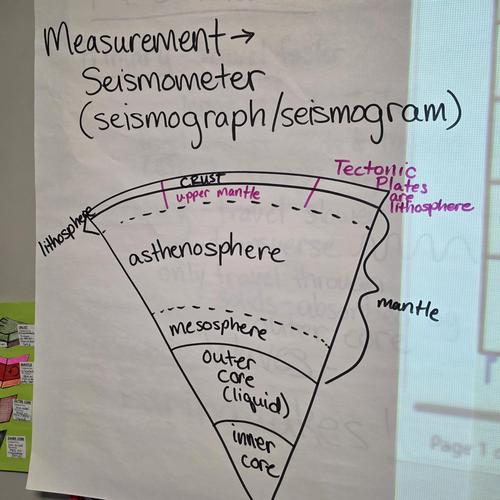
Plate Tectonics Project
Instructions for the Plate Tectonics Project. Due February 26.